Overcome the challenges of cell expansion and product preservation throughout CGT processing.
Independent research demonstrates the medium and material used in sample cryopreservation impacts biologic viability. Formulation must be sterile, free from adventitious agents and qualified before use to remove susceptibility from batch-to-batch. CryoStor® and HypoThermosol® solutions are recognized as the gold standard for freeze and storage media, embedded in over 500 customer clinical applications, and our pathogen reduced human platelet lysates (hPL) provide you with a media that reduces risks and improves downstream cell performance.
CELL PERFORMANCE SOLUTIONS

Human Platelet Lysates
Our pathogen reduced human platelet lysates (hPL) provide you with a media that reduces risks and improves downstream performance over FBS and other chemically defined media. We provide a range of products depending on the regulatory and packaging needs of your processes. And with our robust supply, you’ll have a media that not only performs – but is available when you need it most.
Stemulate®
Platelet Lysate
Stemulate™ is a Xeno- and heparin-free pooled platelet lysate that is used globally in clinical stem cell and immuno-oncology applications.

nLiven PR™
Platelet Lysate
nLiven PR™ is a GMP-ready pathogen reduced human platelet lysate with superior performance and quality standards.

T-Liven PR™
Platelet Lysate
T-Liven PR™ is a GMP-ready human platelet lysate for closed system bioprocessing with release against a T cell growth release assay for optimal immune cell quality.

CELL PROCESSING & HANDLING SOLUTIONS

Sexton Biotechnologies
Signata CT-5™
Sexton Biotechnologies
The Signata CT-5™ allows users to incorporate automation early in the manufacturing process without eliminating critical flexibility during process development. The system provides multiple consumable options for various manufacturing techniques to add safety and control to CGT manufacturing.

CellSeal® Cryogenic Vials
Sexton Biotechnologies
CellSeal® Cryogenic Vials are the first purpose-built rigid containers used in the cell and gene therapy industry with hermetically sealed access ports that withstand the challenges of -196°C storage without compromising CCI.

Biopreservation SOLUTIONS
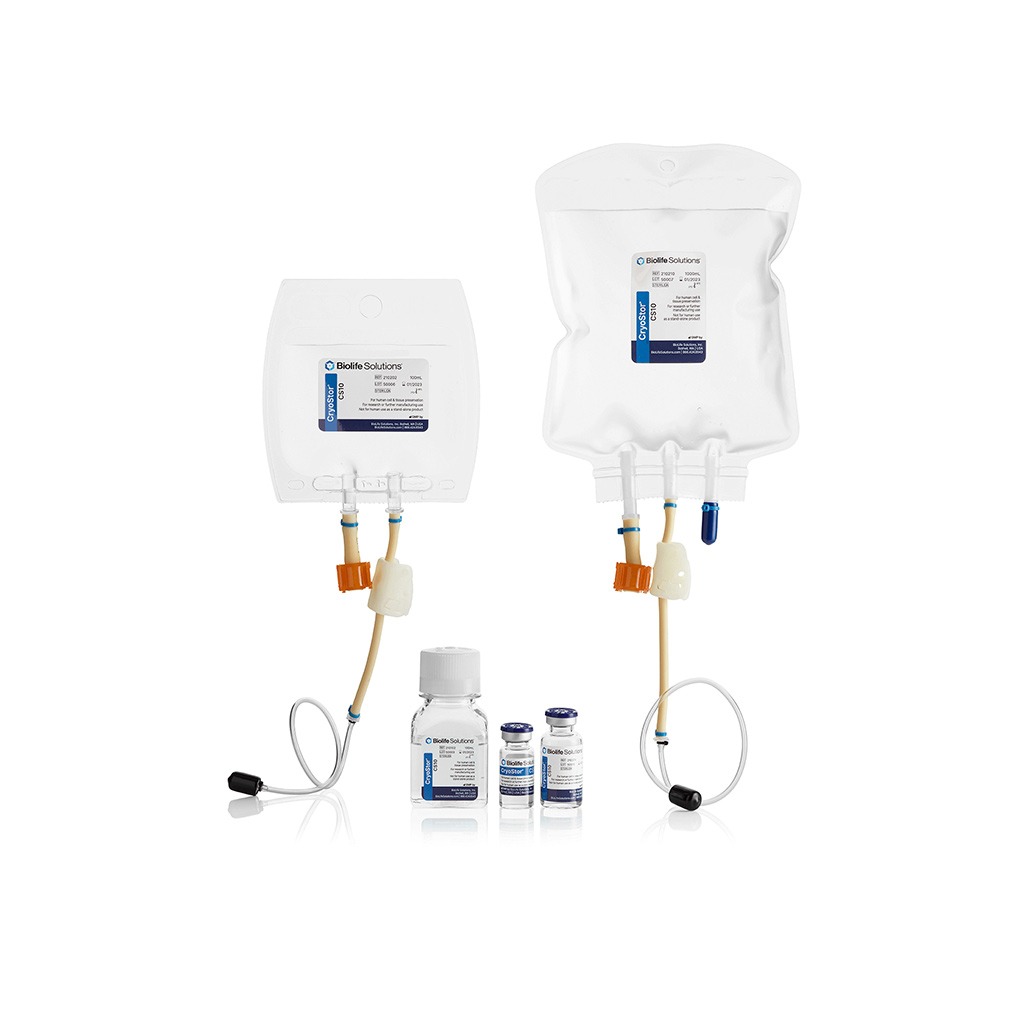
CryoStor®
PROPRIETARY FREEZE MEDIA PRODUCTS
CryoStor® proprietary freeze media products
are intended for cryopreservation of biologics at -70 to -196°C. Across a broad spectrum of cell types, CryoStor formulations have proven more effective in reducing post-preservation apoptosis and necrosis than commercial and home-brew isotonic and extracellular formulations.
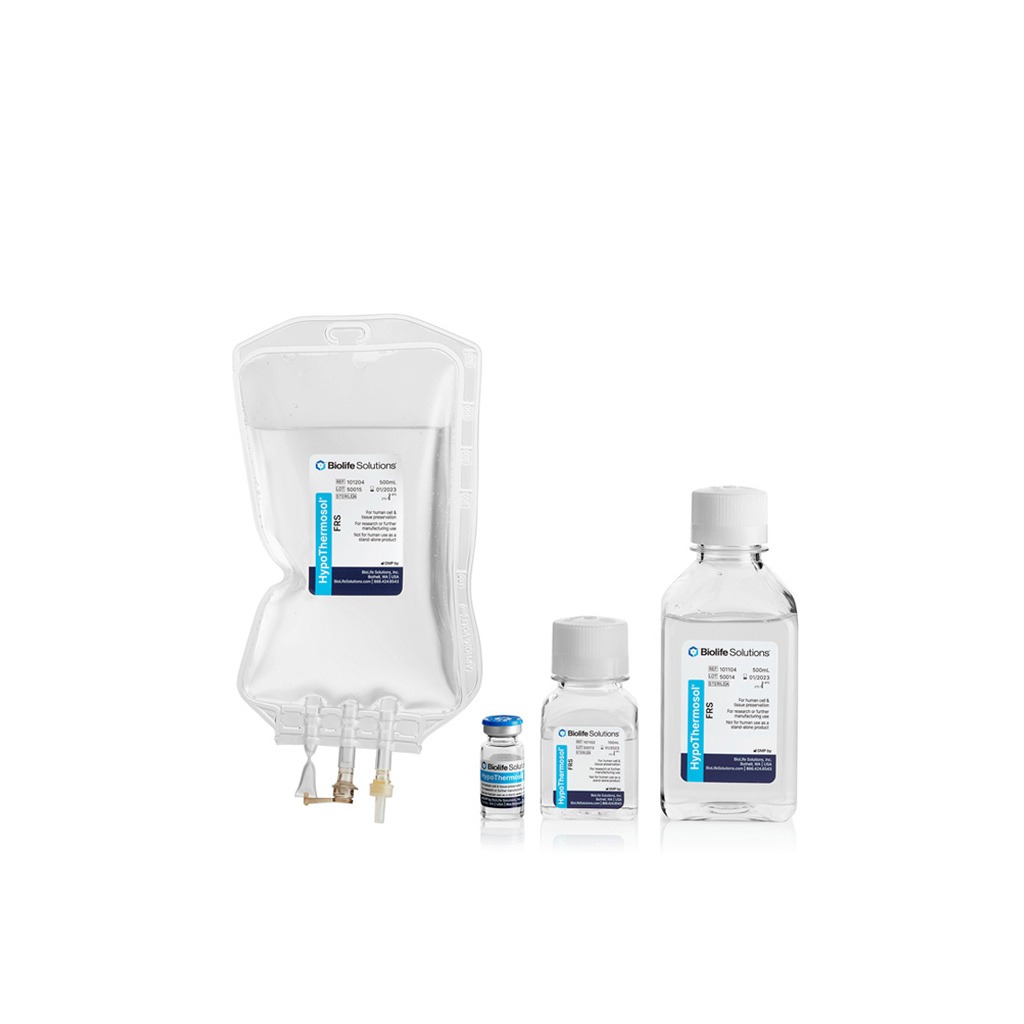
HypoThermosol®
NON-FROZEN STORAGE
HypoThermosol® FRS (HTS-FRS) biopreservation media is a novel, engineered, optimized hypothermic storage and shipping media. Serum-free, protein-free HypoThermosol is designed to provide maximum storage and shipping stability for cells and tissues at 2-8°C.
BloodStor®
CRYOPRESERVE CELLS
BloodStor® is a series of generic cGMP freeze media products used to cryopreserve cells and cell components isolated from umbilical cord blood, peripheral blood, bone marrow.

Thawing Media
CELL AND TISSUE THAWING
BioLife’s Cell Thawing Media products are specifically designed for thawing human cells and tissues following cryopreservation.
