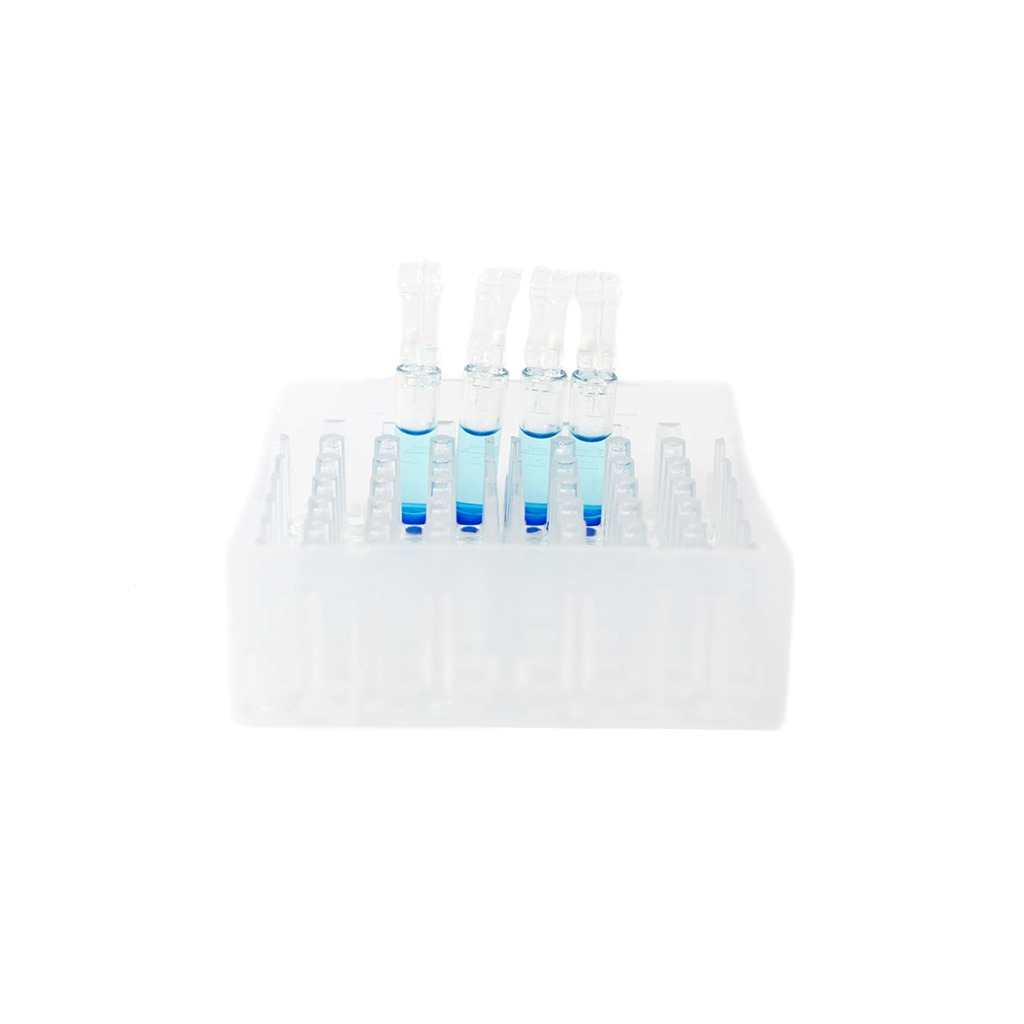
CellSeal® Cryogenic Vials
Closed-system cryogenic vials for CGT manufacturing
CellSeal and CellSeal Clear Access Cryogenic Vials

CellSeal® Cryogenic Vials are the first purpose-built rigid containers used in the cell and gene therapy industry. Unlike traditional screw cap vials and stoppered vials, the hermetically sealed access ports withstand the challenges of -196°C storage without compromising CCI.
With a full suite of accessories and filling systems, vials can be filled manually or with high throughput systems- ideal for both autologous and allogeneic products.
- 5mL and 2mL vial sizes
- Swabbable or capped Luer-compatible fill ports
- USP Class VI compliant materials
- Certificate of Compliance per lot provided with each purchase
- Cryogenic vials can be safely handled when frozen
- Designed for extreme temperature conditions
- Closed-system design prevents sample leakage
- Microbial barrier vent for filling and withdrawal at atmospheric pressure
- Conically-shaped retrieval port for maximum sample recovery
- Loading ports configured for syringes, pipettes, or needleless Luer fittings
- Compatible with the Sexton AF-500 Automated Fill and Seal System and the Signata CT-5 Fluid Management System.
Please download CellSeal resources here:
Frequently
Asked
Questions
All specimen contacting materials are manufactured from USP Class VI compliant materials, and have been subjected to biological safety testing that included genotoxicity, cytotoxicity, sensitization, irritation, hemocompatibility, and acute systemic toxicity.
Yes. A Certificate of Compliance per lot number is provided to the customer with each purchase.
CellSeal Vials have demonstrated low levels of extractables during a leachables study.
The CellSeal Vial has an expiration date of three years from date of manufacture.
Please refer to the corresponding recommended instructions for guidance (IFU) on how to use the CellSeal Vial.
The expansion profile of different solutions are dependent on content, concentration and freezing process. We suggest evaluating the expansion profile of your product in CellSeal Vials under the intended conditions of use. Please contact us for additional information.
CellSeal and CellSeal Clear Access
CellSeal Connect advances the CellSeal platform with a weldable fill and retrieval tube. Designed with viral vector and intermediates in mind, these vials can be filled in a closed system manner and sterile welded onto downstream processing systems.
Compatible with the high-throughput AF-500 fill system, the CellSeal Connect closes out remaining gaps in cell and gene therapy manufacturing.

Radio Frequency Tube Sealing System
Easily access the CellSeal platform using our radio frequency tube sealer. Sealing unit is specifically tuned for optimal sealing of CellSeal vials. Compact system offers consistent and reliable sealing to protect your intermediates or final product.

AF500 Automated
Fill and Seal System
From concept to commercialization, the CealSeal AF-500 delivers the automation difference in a precision engineered and GMP compatible fill-finish system. For cell and gene therapy developers and manufacturers, the AF-500 provides the path to scale up and standardize through increased accuracy, speed, and productivity. Its versatile design for controlled environments include benchtops, biosafety cabinets, or an isolator.

Cellseal Freezing Container
Remove the need for alcohol additives or an external power supply with the CellSeal Freezing Container. Available for 2ml and 5ml CellSeal vials, this unit facilitates controlled freezing through passive cooling at a rate of -1oC/min.


How can we help you?
Get in touch
BioLife Solutions Inc.
3303 Monte Villa Parkway,
Suite 310, Bothell , WA 98021 USA
For assistance, please call us Monday
through Friday (9:00 am to 5:00 pm PST) at:
Toll Free (North America): +1.866.424.6543
Direct: +1.425.402.1400
Fax: +1.425.402.1433