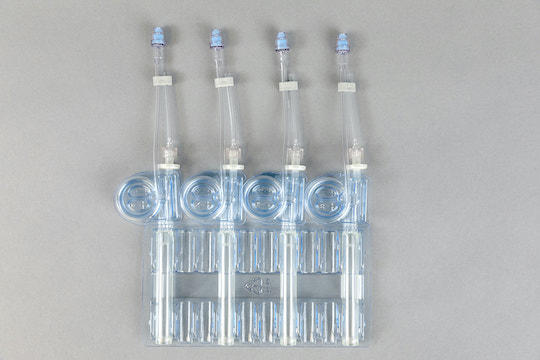
Why CellSeal Cryogenic Vials?
See how CellSeal Cryogenic vials can take you from early stage pre-clinical development all the way through to commercialization.
Integration
There are key items to consider when you start the transition to GMP compliant storage…
- Does the container fulfill quality and regulatory expectations?
- Can the technology be scaled through later-stage development?
- What are the cost implications of implementing a new solution?
CellSeal Cryogenic vials can be implemented and hermetically sealed without significant capital investment. Our vials offer quick and easy startup with the ability scale up to high throughput using automation.
Flexibility
Our solutions offers the ability to embed true flexibility from fill to retrieval…
- Flexible fill ports: fill manually with syringe/pipette/needle and seal with RF Sealing Unit
- Automated solutions: scale accordingly with Signata CT-5 and AF-5oo
- Flexible Retrieval: vial adapters and 18G non-coring needle
- Closed Fill and Retrieval: close out intermediate reagent integration into the manufacturing process with CellSeal Connect
Scalability
Our goal is to provide scalable technology that moves with your development cycle…
- Pre-clinical technology development: low barrier of entry to CellSeal technology with flexible manual fill options and hand-held RF Sealing Unit
- Early-stage technology development: implement closed-system automation strategies as your process moves towards GMP manufacture with the Signata CT-5
- Later stage technology development and commercialization: progress to high-throughput fill/finish automation strategies with the AF-500.